Give the Number of Valence Electrons for Alkali Metals.
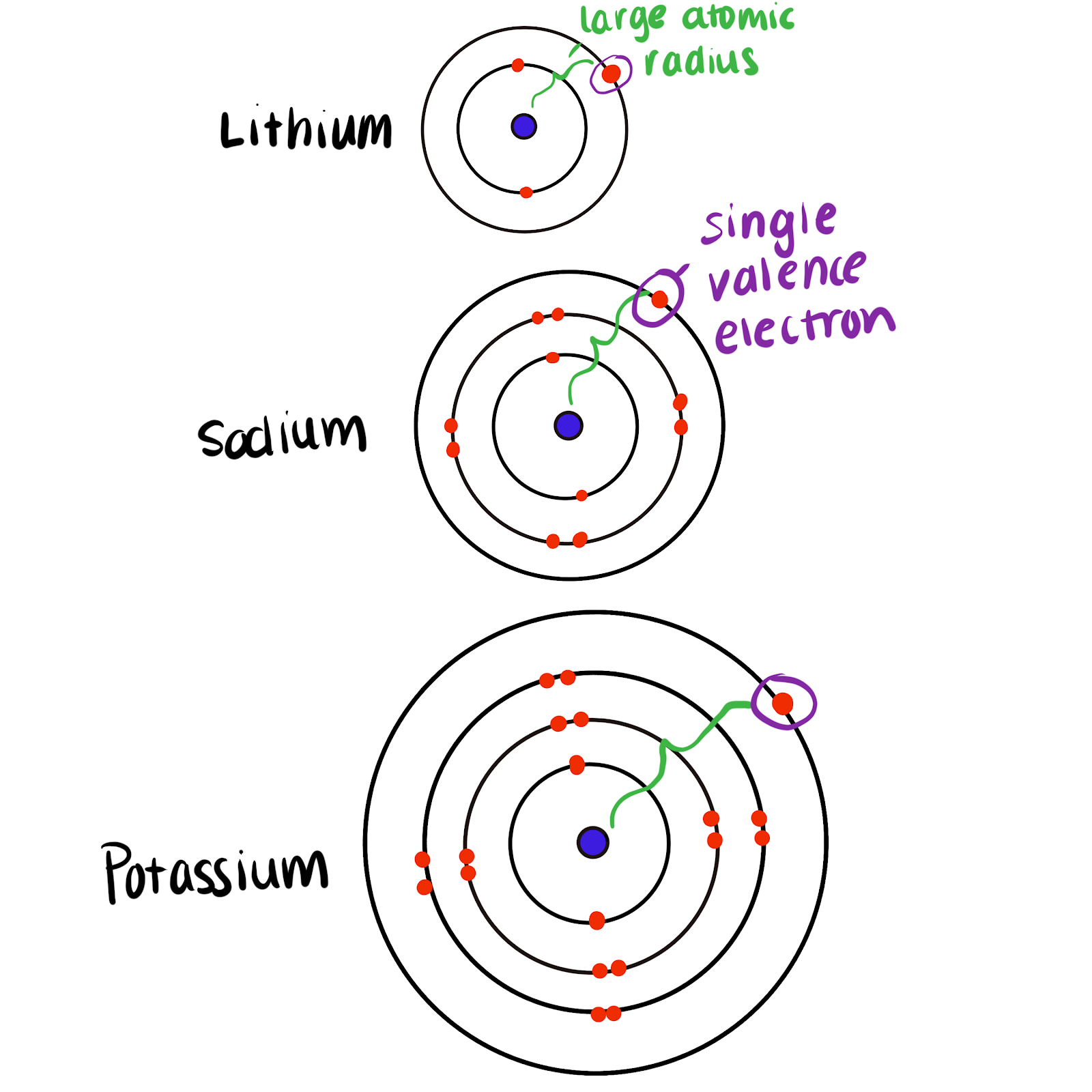
The Group 1 elements in the periodic table are known as the alkali metals. The alkali metals or group 1A family have only one electron in their valance shell.
The halogens Group VIIA were all observed to be colorful reactive elements that combined with oxygen in a 72 atom ratio and with the alkali metals in a 11 atom ratio.
. The ones digit is the number of valence electrons. We review their content and use your feedback to keep the quality high. For sure the Alkali Metals give the best case of group patterns in properties in the periodic table with components displaying the described Homologous conduct.
The main group number for an element can be found from its column on the periodic table. Experts are tested by Chegg as specialists in their subject area. Kason11wd and 4 more users found this answer helpful.
The elements belonging to 1A2A are alkali metals and alkali earth metals whereas the elements belonging to 16th and 17 th are nonmetals. Alkaline earth metals are group 2 elements with 2 valence electrons. Lithium has 3 protons and 1 valence electron.
Since the alkali metals only have one valence electron they typically achieve this state by giving up that electron. This problem has been solved. Chemistry questions and answers.
Valence electrons give us an idea about the ease with which the atoms can form bonds the number of unpaired electrons and how many atoms can take part in a specific chemical reaction. Alkali metals are group 1 elements with 1 valence electrons. The number of valence electrons in alkali metal is 1.
Express your answer as an integer. This is how this works. The number of valence electrons does not depends on the period number.
Similarly for alkaline earth metals general electronic configuration is ns2 there is 2 valence electrons present in the last shell Here elements belonging to group 1 alkali metals with their actual configuration are as follows. This group must be worked out to figure out the number of valence electrons. 3 lithium 1s2.
The most reactive kind of metallic element is an alkali metal of group 1 eg sodium or potassium. The number of valence electrons in groups 1 2 and 13 18. This makes a total of 10 in filled shells leaving only one electron in the outer shell where the valance electrons are located.
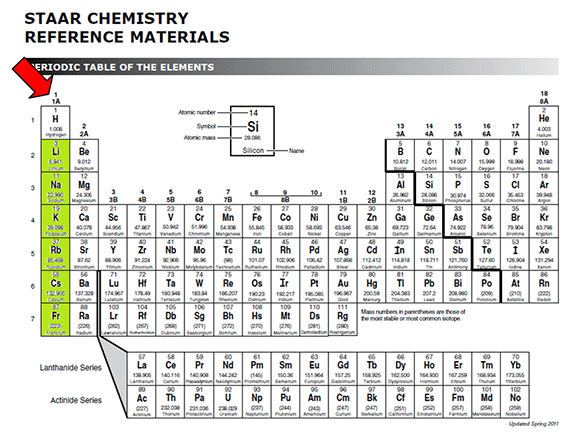
These alkali metals and Hallogens 17th group react very aggressively because 1 A elements has one electron as valency and Hallogens also has valency as onenote valency is slightly different from valency electrons. List the number of valence electrons in each element and classify each element as an alkali metal alkaline earth metal halogen or noble gas. The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized Table PageIndex1.
The Alkali Metals are generally lustrous soft and very reactive Metals at standard temperature pressure and promptly lose their furthest Electron to form cations with charge 1. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of. Problem 60 Medium Difficulty.
In other words the number of valence electrons does not change from up to down but it changes from left to right. Valence Electrons and Ion Formation for the First 20 Elements Element Total Number of Electrons in Neutral Atom Valence Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li. They include lithium sodium and potassium which all react vigorously with water to produce an alkaline solution.
Sodium for example has 11 electrons 2 are in the first filled shell 8 are in the second filled shell. For example carbon is in group 4 and has 4 valence electrons. Alkaline earth metals are the family of metals that belong to group column number 2 in the periodic table.
This is because such an atom has only a single valence electron. Express your answer as an Integer. Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.
In this process the alkali metal is said to be oxidized and whatever takes the electron from the alkali metal is reduced. IN PERIODIC TABLE THERE ARE SIX ELEMENTS IN ALKALI METALS. This group is made up of metals.
There are two ways to find the number of valence electrons in the Alkali Metals. Who are the experts. What is the number of valence electrons.
The first is to use the Periodic Table to figure out how many electrons Alka. Oxygen is in group 6. Give the number of valence electrons for alkali metals.
Give the number of valence electrons for alkali metals. Once quantum mechanics were developed in the 1920s it was found that the alkali metals all had one valence electron thereby explaining their similar reactions and the 21 atom ratio with oxygen. Sodium has 11 protons and 1 valence electron.
Alkali metals Alkali metal any of the six chemical elements that make up Group 1 Ia of the periodic tablenamely lithium Li sodium Na potassium K rubidium Rb cesium Cs and francium Fr. Alkali metals Group 1left rmI right. Alkali metals post-transition metals alkaline earths Li Be.
Stay tuned with BYJUS to learn more about other concepts such as the properties of alkali metals. For neutral atoms the number of valence electrons is equal to the atoms main group number. The answer is b 2 valence electrons.
You can tell the number of valence electrons of the representative elements by the number of column. This group has 2 valence electrons. All the alkali metals have one valence electron.
This group has 3. As all the alkali metals present in the same group so they have same number of valence electrons that is 1. The alkaline earth metals have 2 valence electrons.

Elements Of S Block Properties Of The First Group Elements 1a Alkali Metals In The Periodic Table Science Online
Why Alkali And Alkaline Earth Metals Are Among The Reactive Elements Of The Periodic Table Quora
No comments for "Give the Number of Valence Electrons for Alkali Metals."
Post a Comment